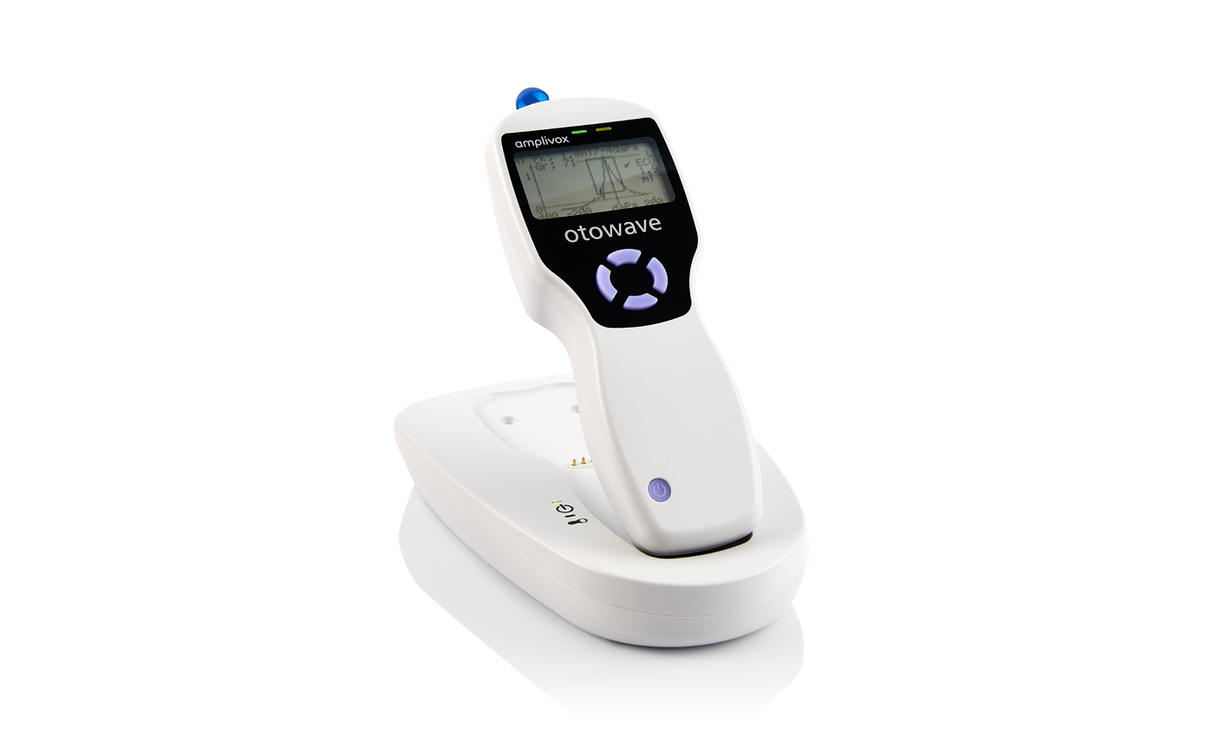
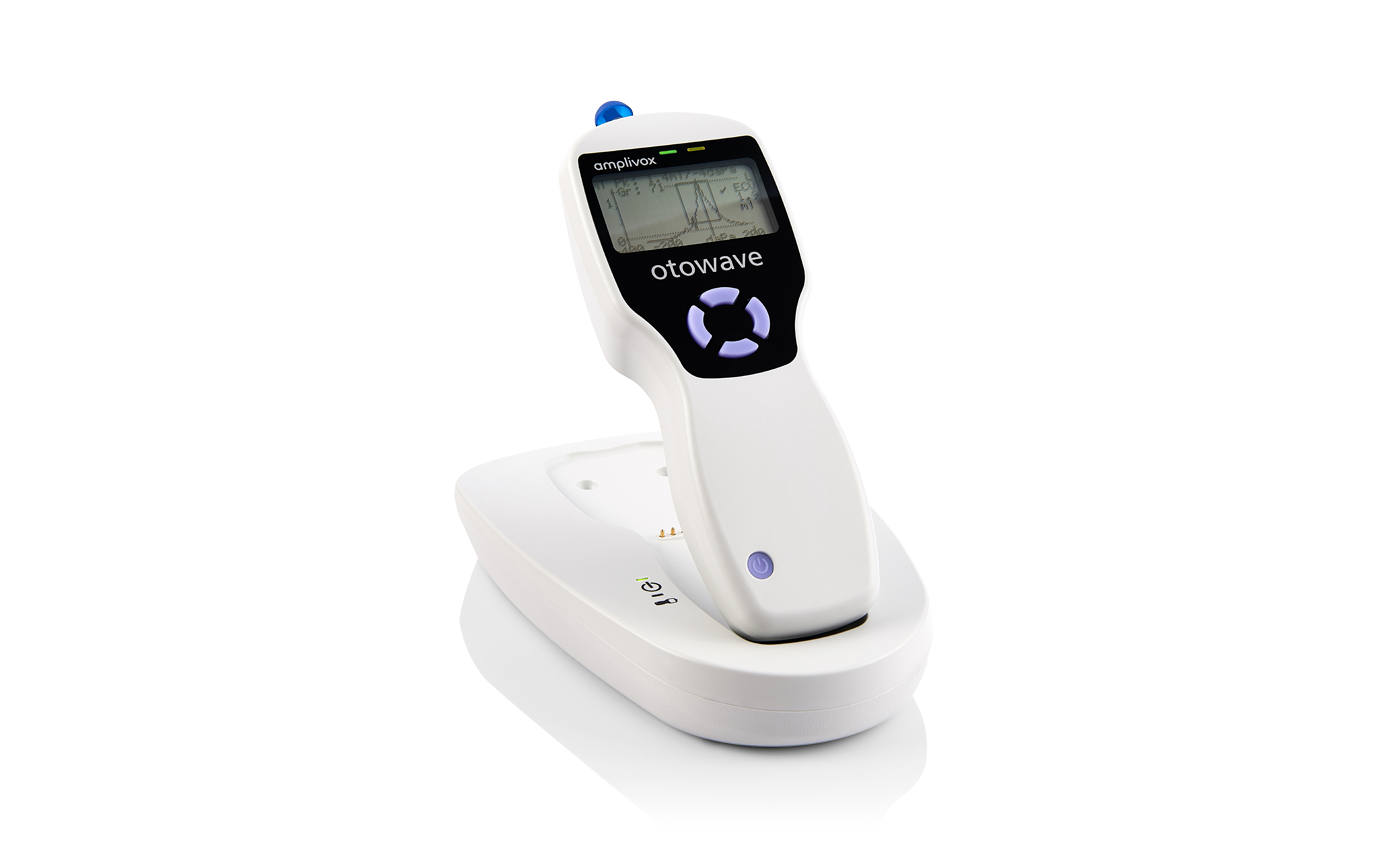
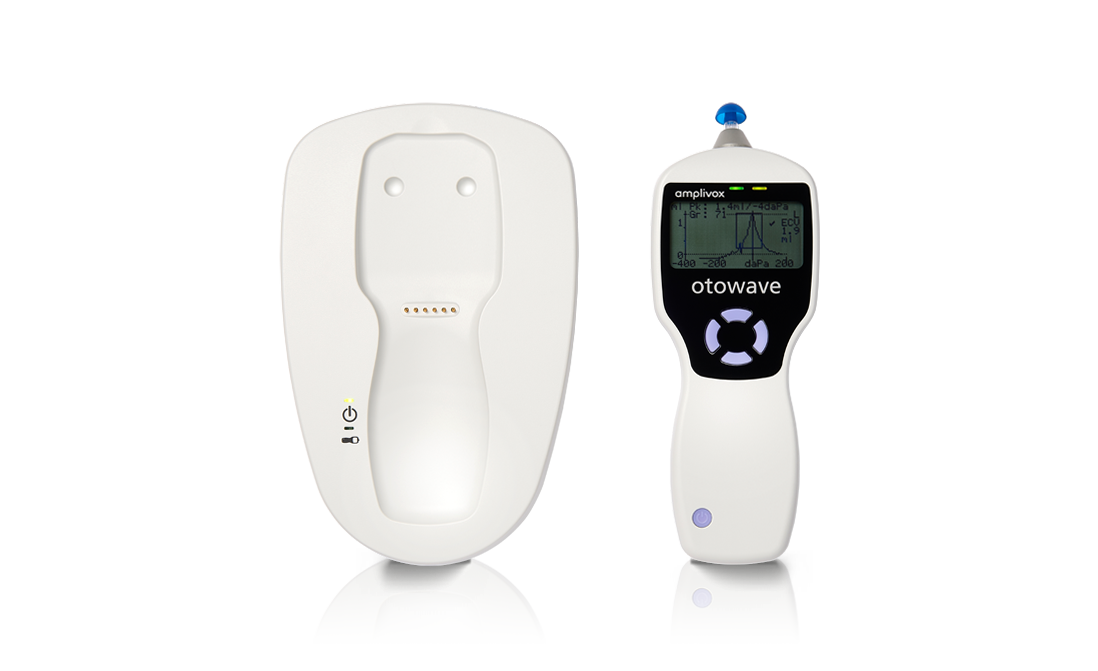
Amplivox Otowave 102-C Handheld Tympanometer with Docking Station
Amplivox Otowave 102-C Handheld Tympanometer with Docking Station may not be in stock. Please call us for ETA before purchasing.
Couldn't load pickup availability
Warranty Information
Warranty Information
3 Years
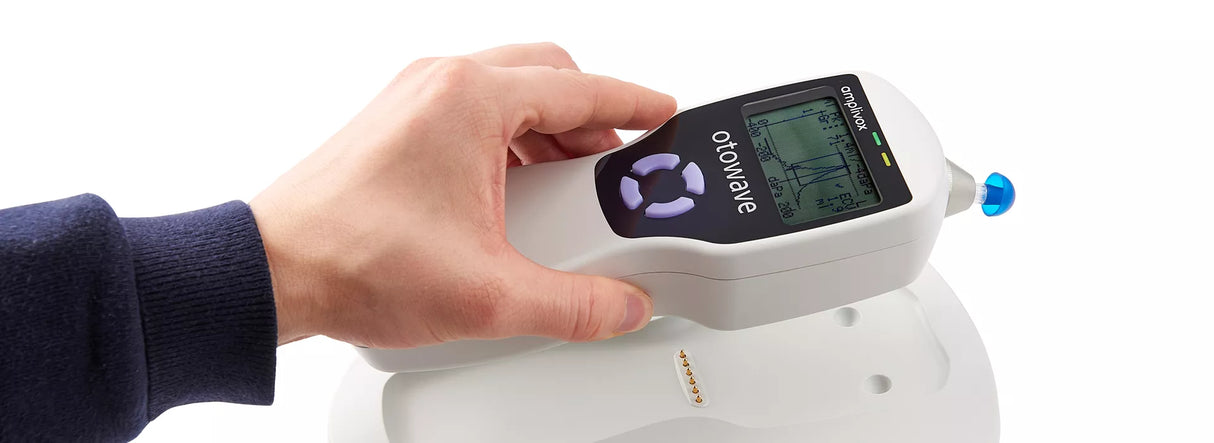
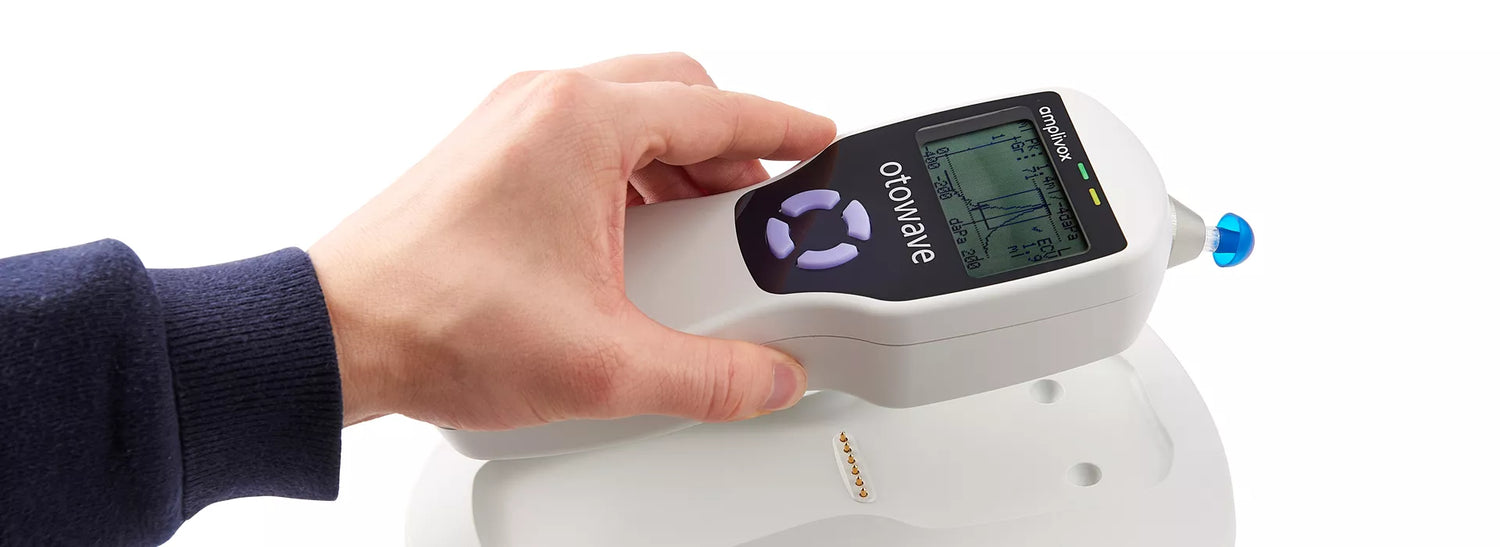
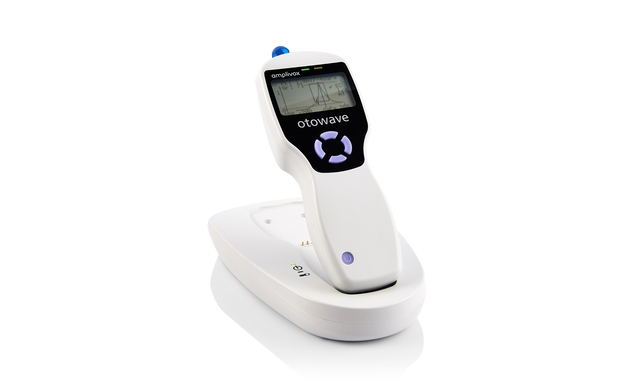
A compact, handheld screening tympanometer featuring a custom-designed docking station for seamless instrument charging and efficient test data transfer, perfectly suited for busy clinical environments.
Intuitive handheld screening tympanometers for reliable and accurate middle ear measurements. The efficiency and ease of use of the Otowave 102 can help to reduce
consultation times, making it the ideal choice for many audiology, ENT and hearing healthcare professionals.
Package Contents
- 1 x Amplivox Otowave Portable Tymopanometer
- 1 x Eartip Selection Box
- 4 x Test Cavities
- Spare Probe Tips and Gaskets
- 1 x Charging Dock Station
- 1 x Carry Case
- Manual & Software (Available Via Website Download)
- 1 x USB Cable (PC Connection)
Key Features
- Portable, handheld design
- 226Hz probe tone
- Live display
- Four ipsilateral reflex frequencies
- Pressure range -400 to +200daPa
- Fast and accurate middle ear measurements
- Intuitive and ergonomic design
- Large LCD graphics display
- Programmable ipsilateral reflex tests
- Optional portable printer and PC interface
- Multiple language options: English, German, French, Spanish, Portugese, Italian, Polish, and Russian
Data Management
The Otowave 102 Tympanometer features an internal memory function, allowing test results to be printed instantly or saved for future review using the included Amplisuite software.
It also provides seamless single-click integration with third-party EMR systems, including Noah, Auditbase, and OtoAccess®, enabling effortless transfer of data and results for enhanced workflow efficiency.
Ease-of-Use
The Otowave 102-C is designed for intuitive operation, providing real-time on-screen information before, during, and after testing. It efficiently measures tympanometry and acoustic reflexes with a live tympanogram display, while also aiding in the identification of ear conditions such as perforated eardrums, otitis media, and cholesteatomas.
Ergonomic Design
Tests are performed with confidence and precision thanks to the Otowave 102-C’s ergonomic design, which fits comfortably in the hand and offers ideal weight distribution. The device ensures an effortless testing experience, featuring a large LCD screen for viewing live tympanograms and a straightforward four-button layout for intuitive operation.
Portability
Weighing only 380g, the simple design allows for complete portability. Travelling audiologists can confidently provide reliable measurements in both mobile and clinic based audiological environments.
Optional Equipment
- Portable Printer
- Infra Red IrDA Cable (PC Connection Required)
- Rechargable Batteries
- Eartips
- OtoAccess Database
Amplisuite
Amplisuite is an audiometry and tympanometry software application which allows for easy results download, processing and management, with the inclusion of useful counselling tools such as speech bananas etc. Providing Auditbase and Noah integration, Amplisuite empowers hearing care personal to review audiological test data and support their patients in the best possible way. Amplisuite is also available as a free stand-alone software option.
| Tympanometry Measurements | |
| Probe tone level | 226Hz ±2%, 85dB SPL ±2 dB |
| Pressure range | +200daPa to -400daPa ±10daPa C5or ±10% (whichever is greater) |
| Direction of sweep | Positive to negative |
| Volumetric range | 0.2ml to 5ml ±0.1ml or ±5% (whichever is greater) |
| Analysis performed | Compliance peak level, compli ance peak pressure level, gradient and equivalent ear canal volume |
| Reflex Measurements | |
| Reflex frequencies | 500Hz, 1kHz, 2kHz, 4kHz ±2% user-selectable |
| Reflex dB range | 85 to 100 dBHL ±3dB (5 or 10dB steps) Threshold measurement or single level |
| Measurement range | 0.01ml to 0.5ml ±0.01ml |
| Analysis performed | Reflex maximum amplitude and pass/ fail at each test level |
| Data Management | |
| Internal database | 32 patient records |
| Optional printer | Thermal printer |
| Data transfer | Via USB cable to Amplisuite, Auditbase, Noah, OtoAccess, and other EMR systems |
| Languages | English, German, Italian, Spanish, French, Portugese |
| Physical Data | |
| Power (battery pack) | NiMH rechargeable battery |
| Dimensions (L x W x H) | 230 x 115 x 70mm |
| Weight | 380g handset / 650 with dock |
| Safety and Standards | |
| Safety | IEC 60601-1 (plus UL, CSA & EN deviations) NiMH rechargeable battery |
| EMC | IEC 60601-1-2 |
| Impedance | IEC 60645-5, Type 2 Tympanometer, ANSI S3.39, Type 2 |
| CE Mark | Complies to EU Medical Device Regulation (MDR 2017/745) |
Payment & Security
Payment methods
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.