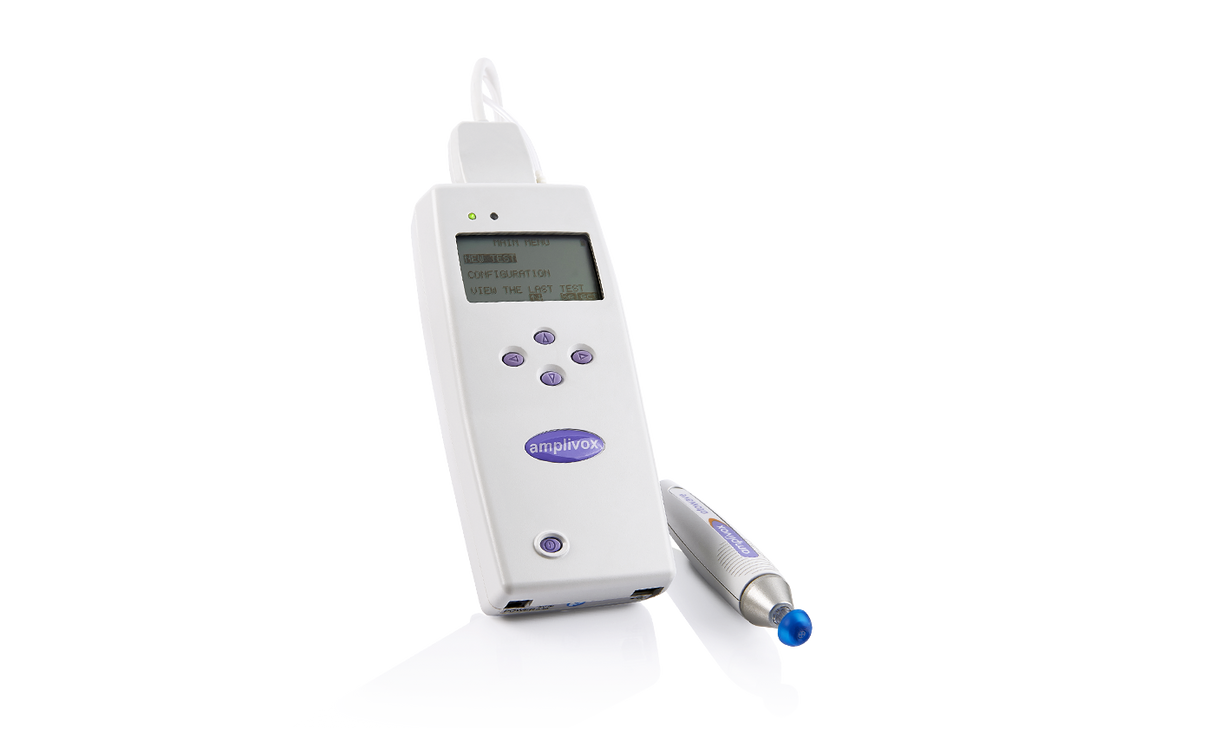
Amplivox Otowave 202 & 202-H Handheld Diagnostic Tympanometer
Amplivox Otowave 202 & 202-H Handheld Diagnostic Tympanometer - Otowave 202 may not be in stock. Please call us for ETA before purchasing.
Couldn't load pickup availability
Warranty Information
Warranty Information
Device - 3 Years.
The Amplivox 202 and 202-H are handheld diagnostic tympanometers that provide fast and accurate middle ear measurements in all age groups including neonates. This is the ideal choice for audiology, ENT, and hearing care professionals seeking a portable tympanometer suitable for both mobile and clinical testing environments. The Otowave 202 and 202-H also offer a wide range of test functionality to ensure testing requirements can not only be met, but exceeded.
Package Contents
- 1 x Amplivox Otowave Tympanometer (202 or 202-H)
- 1 x Eartip Selection Box
- 1 x Contralateral Transducer
- 4 x Test Cavities
- Spare Probe Tips and Gaskets
- 4 x AA Batteries
- 1 x Carry Case
- 1 x Power Supply
- 1 x USB Cable (PC Connection)
- 1 x Manual & Software
Key Features
- Designed for handheld and desktop use
- Mains and battery power functionality
- 226Hz probe tone
- Optional 1kHz probe tone (202-H option) with admittance, susceptance and conductance measurements (YBG)
- Live display
- Enhanced Ipsilateral and contralateral reflex frequencies (0.5, 1, 2 and 4kHz)
- Enhanced mode reflex test
- Auto pass/refer evaluation
- Fully customisable test protocol
- User selectable measurement speeds
- Internal memory, connection to portable printer and/or PC
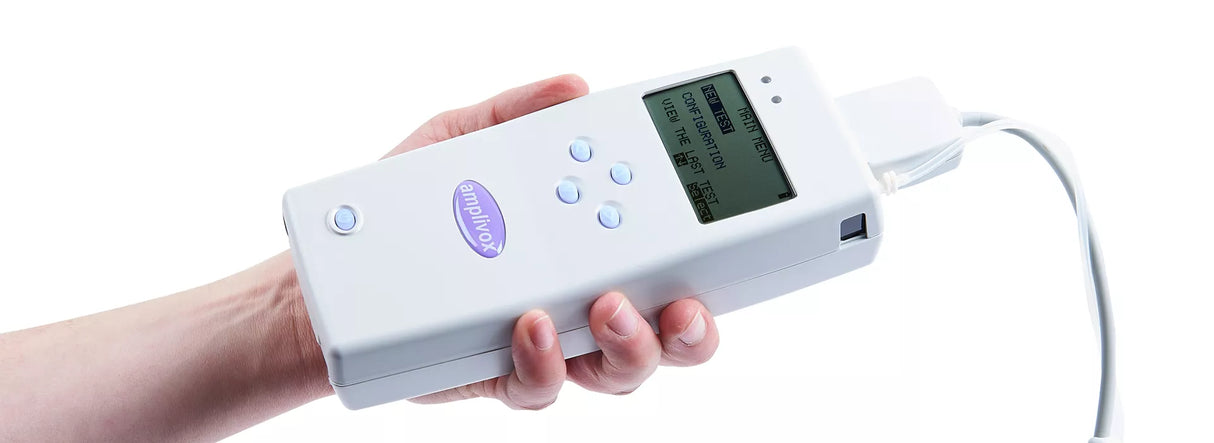
Designed for Small Ears
The Otowave 202 is specifically engineered to deliver fast and accurate middle ear measurements for neonates and children. Its specialised probe, designed for small ear canals, ensures precise results. Diagnostic information is displayed during and after the test, with customisable settings to meet each patient's requirements.
Data Management
Equipped with an internal memory function, test results can be printed instantly or saved for future processing using the included Amplisuite software. The tympanometer also supports single-click integration with third-party Electronic Medical Record (EMR) systems, including Auditbase, NOAH, and OtoAccess®, ensuring seamless data transfer and optimised workflow efficiency.
Portability
Weighing just 430g with batteries, the Otowave 202 is lightweight and easy to carry. Its simple design and dual power options—AA batteries or mains power—make it ideal for both mobile and space-restricted clinical settings.
Comprehensive Testing
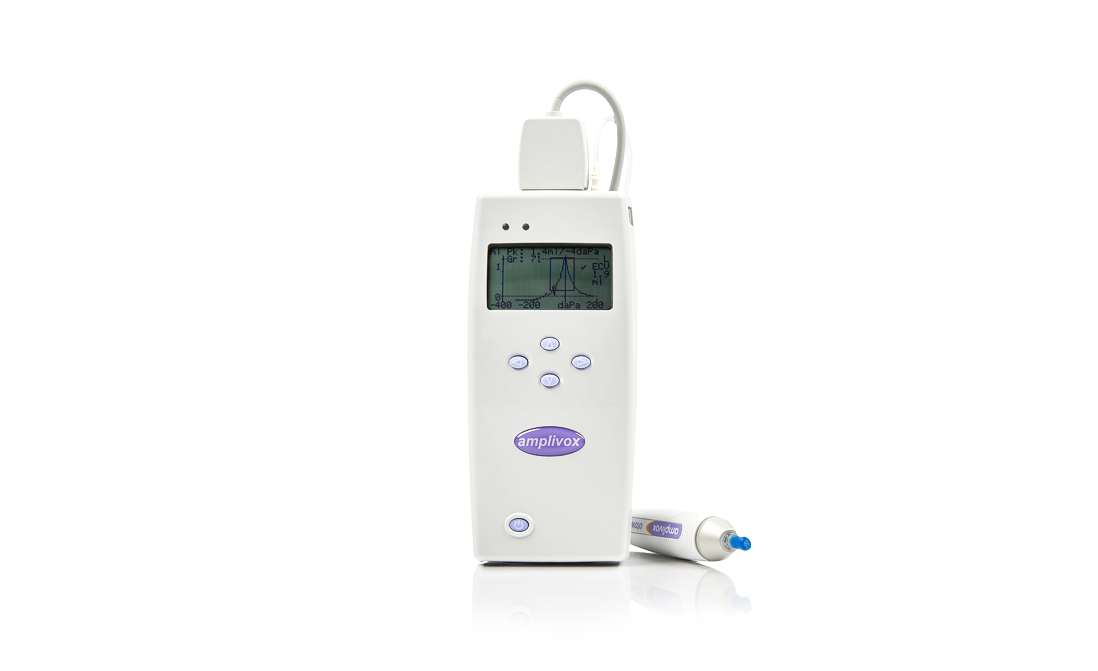
The Otowave 202 provides 226Hz probe tone impedance measurements while the 202-H features a more comprehensive range of diagnostic test functions including user defined 226Hz and 1000Hz probe tone impedance measurements.
The high frequency testing option of 1000Hz tympanometry makes this device ideal for assessing new-borns. When using the Otowave 202-H option in 1000 Hz probe tone mode, users can select a preferred display by pressing the probe function key to switch between the following modes:
- Scalar mode: a traditional admittance tympanogram, as above (Y only compensation)
- Vector mode: a tympanogram displaying B-G compensation
- Component mode: the separate admittance (Y), susceptance (B) and conductance (G) measurements are displayed for clinical review
- User-defined ipsi and contralateral reflex test measurements can be added to the test sequence to ensure a comprehensive test routine. This user programmable acoustic reflex function includes reflex frequency selection, levels and sensitivity
Both devices offer a user programmable range of both ipsi and contralateral reflex test measurements at 500Hz, 1kHz, 2kHz, and 4kHz.
Optional Equipment
- Portable Printer
- Rechargable Batteries
- Eartips
- Power Bank USB Cable
- OtoAccess Database
Amplisuite
Amplisuite is an audiometry and tympanometry software application which allows for easy results download, processing and management. Providing Auditbase and Noah integration, Amplisuite empowers hearing care personal to review audiological test data and support their patients in the best possible way. Amplisuite is also available as a free stand-alone software option.
| Tympanometry Measurements | |
| Probe tone level | 226Hz ±2%, 85dB SPL ±2 dB 1000Hz ±2%, 79dBSPL ±2dB (202-H only) |
| Pressure range | +200daPa to -400daPa ±10daPa or ±10% C5(whichever is greater) |
| Direction of sweep | Positive to negative |
| Volumetric range | 226Hz: 0.2ml to 5ml ±0.1ml or ±5% (whichever is greater) 1000Hz: 0.1ml to 5ml ±0.1ml or ±5% (whichever is greater) |
| Sweep speeds | Selectable: 100, 200 or 300daPa/sec |
| Analysis performed | Admittance peak level in ml (226Hz) or mΩ (1000Hz) & pressure at peak, gradient in daPa (for 226Hz) and ear canal volume (ECV) @ 200daPa |
| Reflex Measurements | |
| Reflex type | Ipsilateral, contralateral or both |
| Reflex frequencies | Ipsilateral and contralateral: 500Hz, 1kHz, 2kHz & 4kHz (±2%) user-selectable |
| Reflex levels | Ipsilateral: 70dBHL to 100dBHL ±3dB (5 or 10dB steps) Contralateral: 70dBHL to 110dBHL ±3dB (5 or 10dB steps) Threshold measurement or single level |
| Reflex direction threshold | 0.01ml to 0.5ml ±0.01ml (configurable in 0.01ml steps) |
| Analysis performed | Reflex maximum amplitude and pass/fail at each test level |
| Data Management | |
| Internal database | 18 patient records |
| Optional printer | Thermal printer |
| Data transfer | Via USB cable to Amplisuite, Noah, OtoAccess® and other EMR systems |
| Languages | English, German, Italian, Spanish, French, Portuguese |
| Physical Data | |
| Power | Mains: 100-240Vac; 50/60Hz (approved to medical safety standards) Batteries: 4 x AA (either Alkaline or NiMH, the latter recharged external to the instrument) |
| Dimensions (L x W x H) | Base unit: 190 x 85 x 40mm Probe: 130 long x 25mm diamete |
| Weight | Base unit: 330g (without batteries, using mains power); 430g (with batteries), probe: 115g (including connecting cable) |
| Safety and Standards | |
| Safety | IEC 60601-1 (plus UL, CSA & EN deviations) |
| EMC | IEC 60601-1-2 |
| Performance | IEC 60645-5, Type 2 Tympanometer, ANSI S3.39, Type 2 |
| CE Mark | Complies to EU Medical Device Regulation (MDR 2017/745) |
Payment & Security
Payment methods
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.