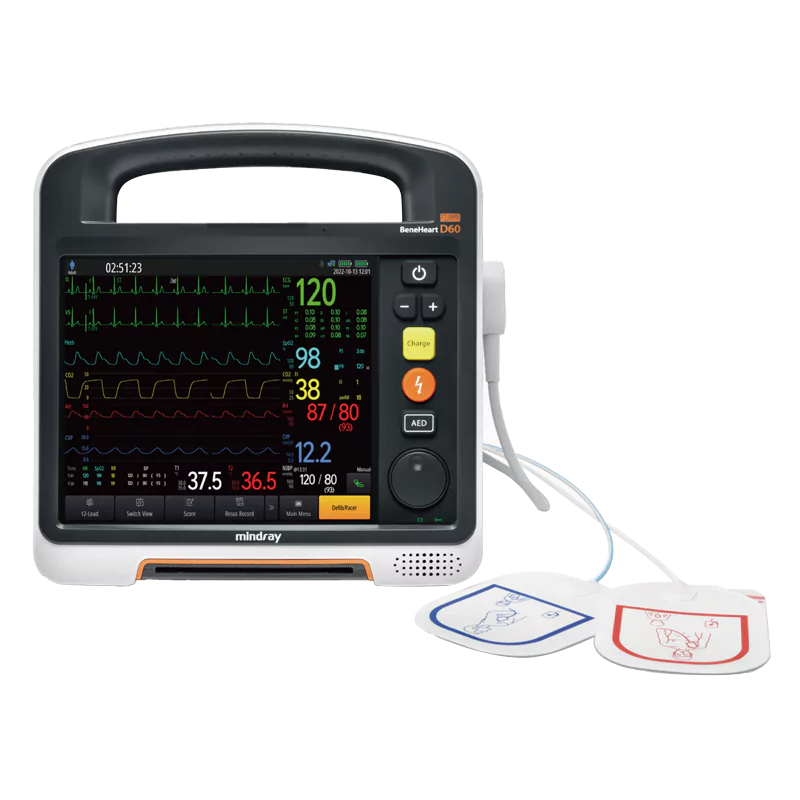
Mindray BeneHeart D60 Defibrillator and Monitor
Mindray BeneHeart D60 Defibrillator and Monitor may not be in stock. Please call us for ETA before purchasing.
Couldn't load pickup availability
Improve the quality and effectiveness of cardiopulmonary resuscitation with the next-generation Mindray BeneHeart D60 defibrillator. Its new QShockTM™ technology takes less than 5 seconds from device power on to shock, ensuring timely response. Analyze rescue data to identify training needs and improve survival rates. The three-in-one solution guarantees high-quality rescue for SCA patients.
All In One
The BeneHeart D60 combines multiple essential functions into a single device, including 360J Manual Defib, AED, Pacing, and Monitoring Capabilities: ECG, SPO2, NIBP, CO2, IBP, TEMP.
One Device with Many Talents
In unpredictable rescue situations, providing on-site diagnosis and analysis for patients is crucial for immediate treatment, but carrying multiple devices can be burdensome. That's why we've equipped the D60 with advanced diagnostic and analytical technologies. It’s more than just a defibrillator and monitor; it also functions as a 12-lead ECG machine, a point-of-care ultrasound, an infrared ear thermometer, and includes scoring and early warning tools.
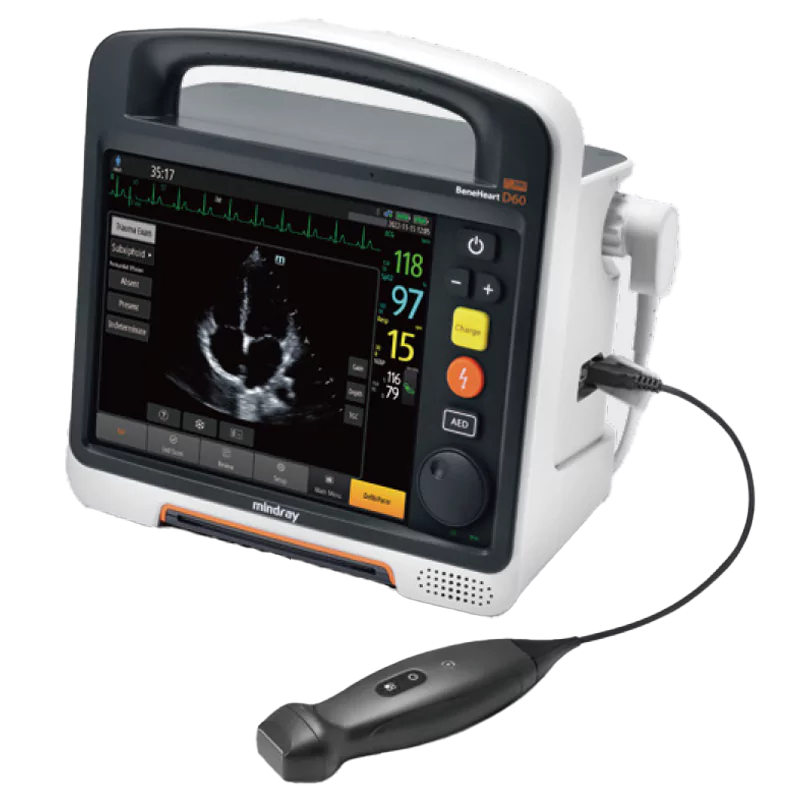
Point-of-Care Ultrasound (POCUS)
- Step-by-step trauma identification (FAST/eFAST)
- Reference images help quickly detect abnormalities by scanning comparison
- Operation guides show how to properly place the probe
- High-quality images with excellent resolution for accurate decision-making
- Supports heart, lung, and abnormal examinations. There's no need to carry extra probes

Professional 12-Lead ECG Analysis
- Special 12-lead ECG accessories: integrated 4 limb leads + removable 6 chest leads, more flexible for both monitoring and resting analysis.
- Serial comparison of two ECG reports on one screen allows to easily observe abnormal changes
- Critical value & MI location help to quickly judge the risk and situation of patients with chest pain
- Comparing two ECG reports on one screen allows to easily observe abnormal changes

Scoring & Early Warning Tools
- Supports GCS/EWS/HEART scoring
- Help to rapidly obtain the severity of the patient and detect the risk of deterioration in time
- TBI (Traumatic Brain Injury) warning combined with GCS and vital signs (SPO2, ETCO2, SBP) monitoring
- Effective management and timely treatment of TBI patients to ensure better prognosis

Rescue Triangle (Resuscitation, Debriefing, Training)
To ensure high-quality and high success rates of cardiopulmonary resuscitation (CPR), the resuscitation team must undergo regular review and analysis of rescue data. This can help identify areas for improvement through routine training and assessment. Combining resuscitation, debriefing, and training is essential for advancing rescue quality and increasing the survival rate of sudden cardiac arrest (SCA) patients. The BeneHeart D30 combines the three key aspects of the rescue triangle into one device.
Faster Resuscitation
-
QShock™ Faster To Shock: In line with the BeneHeart series, D30 is also equipped with new QShock™ technology, taking less than 5s
from power on to shock.

- Shorter Interruption: Mindray has developed state-of-the-art technologies that can filter out the compression interference for both ALS and BLS, to effectively reduce pauses in recognizing heart rhythm*.

-
CPR Feedback of Both Process & Effect: With the CPR process feedback monitoring the real-time performance of rescuers, and the effect evaluation
reflecting patients' responses to the rescue, D30 provides a comprehensive assessment of CPR quality to obtain a satisfactory resuscitation result.
* only available when you perform CPR using defib pad or the CPR sensor

Structured Debriefing
D30's structured debriefing protocols improve the performance of resuscitation teams in subsequent resuscitation events.
- The key data of each rescue is automatically uploaded to the debriefing system
- CPR quality and defibrillation data included
- Not only post-event analysis, but also periodic review

Hands-On Training
- Training mode in D30 helps you get real operation experience
- Both single and multi-person modes are available
- Supports CPR and defib operation training
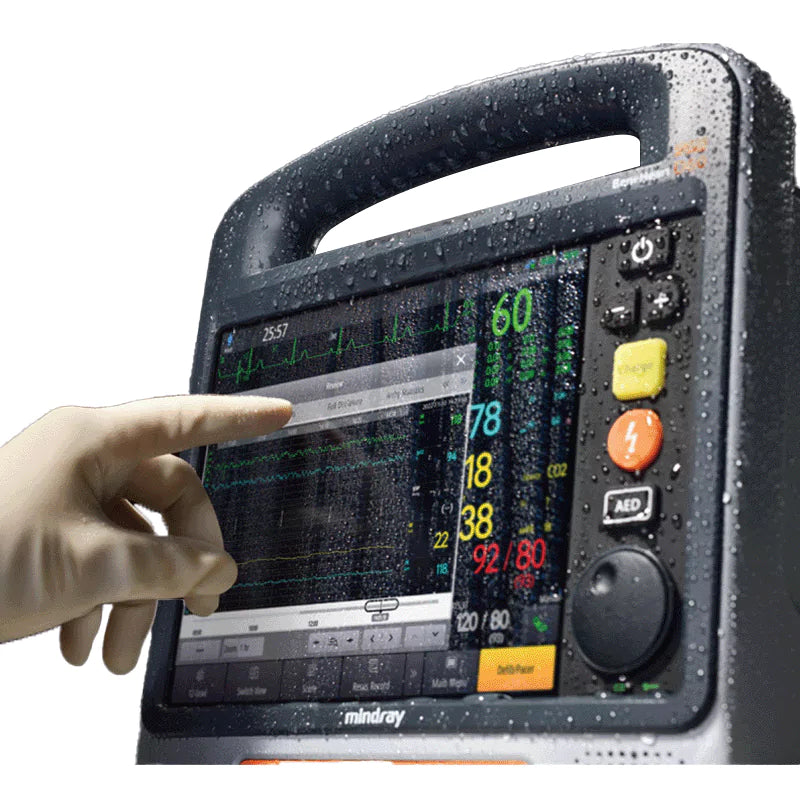
High Definition Capacitive Touch Screen Combined With Physical Knob and Buttons
The D60 features advanced high-definition capacitive touch screen technology and a sleek flat UI design. The 9” display boasts a 1200×1020 resolution with auto-brightness for optimal visibility in any setting. Enjoy confident operation with a gesture-control touchscreen, complemented by physical knobs and buttons for key functions. Designed for wide adaptability, the D60 remains fully operational even when the screen is wet or when you're wearing up to 5 layers of gloves, thanks to its intelligent sensing capabilities.
Visual AlarmSight™
The D60 provides a series of problem-solving support with graphical visualisation, not only alerting the you to the problems, but also suggesting solutions to help resolve them.

Refines Toughness
Prepared for varied scenarios and contingencies, D30 is designed to take on every new challenge in hospital environments anytime, anywhere. D30 empowers clinicians to respond confidently to changing clinical requirements and stay prepared for whatever comes their way.
- Meets all standards for helicopter and other transport
- Operates in temperatures ranging from -20°C up to +55°C
- 1.5m drop protection without carry case, and 3m with carry case
- IP55 water/dust-proof
- Bend-resistant cable, withstanding more than 400,000 bending tests and extremely tight interface connection withstanding 100kg pulling force
- Resistant to 49 disinfectants
- Thick, ultra-durable carry case made of special ballistic nylon fabric which is often used in flak jackets
- Touch screen protected by the latest version of Corning®'s tough glass
M-Connect™ IT Solution and M-IoT Device Manager
In the past, it has been difficult to effectively and comprehensively manage rich information from defibrillators. The M-Connect™ IT solution enables data transmission between remove vehicles to in-hospital departments in real-time, helping medical staff prepare and diagnose in advance.
Pre-Arrival Clinical Data
Pre-arrival patient clinical data such as real-time vital signs, 12 lead-report, and ultrasound report can be sent to the target hospital when the patient is in transport.

Flexible Data Transfer
The D60 is not only just capable of sending patient data to the CMS service remotely via 4G, but it can also connect to the medical pad via Wi-Fi or Bluetooth to send the data to the ePCR system.
M-Connect™ IT Solution integrates the patient data from D60 to avoid manual recording errors and allows flexible patient data viewing on mobile devices anytime, anywhere. Its simple yet robust network connection is compatible with the standard information infrastructure found in most hospitals.

M-IoT device manager obtains comprehensive device data to help biomedical engineers ensure the safety and effectiveness of all equipment at all times.
- Real-time failure monitoring and guidance for timely maintenance, reducing equipment downtime
- Battery status monitoring to limit interruptions
- IP/MAC address for network access control to ensure cybersecurity

Additional Features
- Ultra-Light: only 4.2kg with battery.
- Magnetic Connector: automatically attracted to connect, no precise alignment is needed.
-
Versatile Paddles: external paddles with contact indicator to improve usability for clinicians.
- Status Indicator: clear status at a glance with comprehensive auto-test.
- 6-Channel Printer: paper width 110mm for up to 6 curves.
| Physical Specifications | |
| Dimension | Paddle version: L275xH280xW160mm without external paddles Pad version: L275xH280xW155mm |
| Weight | 4.3 kg (the equipment is configured with AC power input, 3/5-lead ECG and manual defibrillation) 3.9 kg (the equipment is configured with DC power input, 3/5-lead ECG, manual defibrillation but without the paddle tray) |
| Environmental and Physical Requirements | |
| Water resistance | IPX5 |
| Solids resistance | IP5X |
| Temperature | Operating: -20 to 55°C Storage: -40 to 75°C |
| Humidity | Operating/storage: 5 to 95% (non-condensing) |
| Altitude | Operating/storage : -382m to +4575 m |
| Shock | Meets the requirements for medical devices of 6.3.4.2, EN1789 (10.1.3, IEC60601-1-12), RTCA-DO-160G-2010, Section 7 |
| Vibration | Meets the requirements for medical devices of 6.3.4.2, EN1789 (10.1.3, IEC60601-1-12) 10.1.4, IEC60601-1-12, MIL-STD-810G, method 514.6, helicopter-category 14 and ground vehicle-category 20 |
| Bump | Meets the requirements of 6.3.4.2, EN1789 |
| Free fall | 1 fall on each surface (6 surfaces in total), at the height of 1.5 m 1 fall from the normal operation position of the equipment configured with a carry case, at the height of 3.0 m |
| EMC | Meets IEC60601-1-2 |
| Safety | Meets EN/IEC 60601-1 |
| Display | |
| Type | LCD color capacitive touch display, protected by tempered glass |
| Dimensions | 9 in |
| Resolution | 1200 x 1020 pixels |
| Display waveforms | Max. 7 channels |
| Wave viewing time | Max. 36 s (ECG) |
| Sweep speed | ECG/SPO2: 6.25, 12.5, 25, 50mm/s RESP/CO2: 3, 6.25, 12.5, 25, 50mm/s |
| Trace freeze | Yes |
| Screenshot | Yes |
| High contrastmode | Yes |
| Auto-brightness | Yes |
| Gesture control | Yes |
| Power | |
| AC power | |
| Line voltage | 100 to 240 V |
| Current | 1.8 to 0.8 A |
| Frequency | 50/60 Hz |
| DC power (with DC/AC inverter) | |
| Input voltage | 18 V 12-30.3V, with transport dock |
| Input current | 7.2 Amax 15.5 to 6.5A, with transport dock |
| Battery | |
| Type | 4500 mAh, rechargeable lithium ion battery pack |
| Number | DC version: max. 2 AC version: max. 1 |
| Charge time | Less than 3 hours to 90% and less than 4 hours to 100% with equipment power off |
| Capacity indicator | 5-segment led indicator for fast battery capacity evaluation |
| Capacity (new, fully charged battery) | Monitoring mode: 6.5 hours, configured with 3-/5-lead ECG, manual defibrillation, screen brightness set to the lowest level without printing Defib mode: 220 times, 360 J discharge at intervals of 1 minute without recording Pacing mode: 4.5 hours, 50 Ohm load impedance, pacing rate: 80 bpm, pacing Output: 60 mA |
| Recorder | |
| Method | High-resolution thermal dot array |
| Waveforms | Max. 6 channels |
| Speed | 6.25 mm/s, 12.5 mm/s, 25 mm/s, 50mm/s |
| Paper width | 110 mm |
| Reports | Real-time waveforms, ST real-time, QT real-time, event real-time, physiological alarm, frozen waveforms, tabular trends review, graphic trends review, physiological event review, full disclosure review, 12-lead analysis review, rescue record, event summary, auto test, and configuration |
| Auto recording | Recorder can be configured to record marked events, charge, shock, alarm, auto test |
| Data Storage | |
| Internal storage | 4 GB |
| Events | Up to 1000 events for one patient |
| Waveform storage | Up to 120 hours of consecutive ECG waveform |
| Tabular trends | 200 hours, resolution: 1 min |
| Voice recording | At least 8 hours for each patient |
| Data export | Data can be exported to PC through USB flash memory |
| Defibrillator | |
| Waveform | Biphasic truncated exponential waveform, with impedance compensation |
| Energy accuracy | ±2 J or 10 % of setting, whichever is greater |
| Power on time | Less than 2 seconds with a new, fully charged battery |
| Charge time | Less than 3 seconds to 200 J with a new, fully charged battery |
| Less than 7 seconds to 360 J with a new, fully charged battery | |
| ECG recovery time | Less than 2.5 seconds |
| Shock delivery | Via multifunction defib electrode pads, or paddles |
| Patient impedance | 25 to 300 Ω (external defibrillation) |
| Range | |
| Manual mode | |
| Output energy | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 50, 70, 100, 120, 150, 170, 200, 300, 360 J |
| Synchronous cardioversion | Energy transfer begins within 60ms of the QRS peak Energy transfer begins within 25ms of the external sync pulse |
| AED mode | |
| Output energy | User configurable |
| AED shock series | Energy level: 100 to 360J, configurable for adult; 10 to 200J, configurable for pediatric Shocks: 1, 2, 3, configurable Meets 2020 AHA/2021 ERC guidelines by default |
| Time from rhythm analysis to charge done | Initial analysis: 10s Non-initial analysis: 8s |
| AED mode monitor parameters | ECG, SPO2, CO2, NIBP, filtered ECG, CPR feedback, CCF, CQI |
| Sensitivity and specificity | Meets IEC 60601-2-4 and AHA recommendation |
| Noninvasive Pacing | |
| Waveform | Monophasic square wave pulse |
| Pulse width | 20 ms or 40 ms, ±5% |
| Refractory period | 200 to 300 ms, ±3% (function of rate) |
| Pacing mode | Demand or fixed |
| Pacing rate | 30 ppm to 210 ppm, ±1.5% |
| Pacing output | 0 mA to 200 mA, ±5% or 5mA, whichever is greater |
| 4:1 pacing | Pacing pulse frequency reduced by factor of 4 when activated |
| ECG | |
| Lead type | 3 leads ECG, 5 leads ECG |
| Lead selection | 3-lead: I, II, III 5-lead: I, II, III, aVR, aVL, aVF, V |
| Heart rate display | Adult: 15 to 300 bpm Pediatric: 15 to 350 bpm Neonate: 15 to 350 bpm |
| Resolution | 1 bpm |
| Arrythmia | Yes |
| Alarms | Yes |
| ST/QT monitoring | Yes |
| ECG size | 1.25 mm/mV (x0.125), 2.5 mm/mV (x0.25), 5mm/mV (x0.5), 10 mm/mV (x1), 20 mm/mV (x2), 40 mm/mV (x4), Auto |
| Sweep speed | 6.25 mm/s, 12.5 mm/s, 25 mm/s, 50 mm/s |
| Myocardial infraction (MI) location diagram | Yes |
| Respiration | |
| Method | Trans-thoracic impedance |
| Range | Adult: 0 to 200 rpm |
| Resolution | 1 rpm |
| SpO2 Pulse Oximetry | |
| Mindray SpO2 | |
| Range | 0 to 100% |
| Resolution | 1% |
| PR range | 20 to 300bpm |
| Nellcor SpO2 | |
| Range | 0 to 100% |
| Resolution | 1% |
| PR range | 20 to 300 bpm |
| Masimo SpO2 | |
| Range | 1 to 100% |
| Resolution | 1% |
| PR range | 25 to 240 bpm |
| NIBP | |
| Operating mode | Manual, Auto, STAT, Sequence |
| Static pressure range | 0 to 300mmHg |
| Displayed pressures | Systolic, Diastolic, Mean |
| Cuff inflation pressure (default) | Adult: 160mmHg Pediatric: 140mmHg Neonate: 90mmHg |
| PR range | 30 to 300 bpm |
| CO2 | |
| Sidestream CO2 | |
| Measurement range | 0 to 150 mmHg |
| Resolution | 1 mmHg |
| awRR measurement range | 0 to 150 rpm |
| awRR accuracy | 0 to 60 rpm:±1 rpm 61 to 150 rpm: ±2 rpm |
| Sample flowrate | 50ml/min |
| Temperature | |
| Parameter | T1, T2, TD |
| Range | 0 to 50°C |
| Resolution | 0.1°C |
| Infrared ear temperature | Can be obtained via NFC |
| IBP | |
| Channels | 2 |
| Zero adjustment range | ±200 mmHg |
| Resolution | 1mmHg |
| Sensitivity | 5μV/V/mmHg |
| Measurement Range | -50 to 360mmHg |
| CPR Feedback | |
| Parameters monitored | From CPR sensor*: rate, depth, recoil, compression fraction (CCF), interruption time From pads: rate, interruption time From Mindray SPO2: rate, CCF, interruption time, Compression Quality Index (CQI) |
| CPR metronome | Yes |
| CPR countdown | Yes |
| CPR filter | Yes |
| CPR Sensor* | |
| Weight | Approximately 180 g (without battery) |
| Thickness | 17.5 to 19 mm |
| Compression depth | Measurement range: 0 to 8 cm Accuracy: ±5 mm or 10%, whichever is greater |
| Compression rate | Measurement range: 40 to 160 cpm Accuracy: ±2 cpm |
| Point-of-Care Ultrasound | |
| Probe type | Phased array, 2.0-4.0 MHz |
| probe weight | 260±10 g |
| Appoication | Supports adjusting gain, depth, TGC Supports freezing, playing and saving the images Supports reviewing, printing and sending the reports Provides step-by-step trauma identification, operation guide and reference image |
| Scoring & Warning Tools | |
| Scoring type | GCS, P-GCS score NEWS, MEWS, NEWS2 score HEART score |
| TBI warning | Provides trend and warning prompts for SPO2, EtCO2, SBP and GCS score |
| Network | |
| Data connection | Wired, Wi-Fi, 4G, Bluetooth |
| Data transmission | |
| Patient data | In-hospital: sends real-time data to CMS or HL7 service via Wi-Fi or wired network Pre-hospital: sends real-time data to CMS via 4G network, to third-party ePCR via Bluetooth* (connecting with medical pad) |
| Device data | Sends device data (such as auto test report, battery status, etc.) to the device management system via Wi-Fi or wired network |
Payment & Security
Payment methods
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.